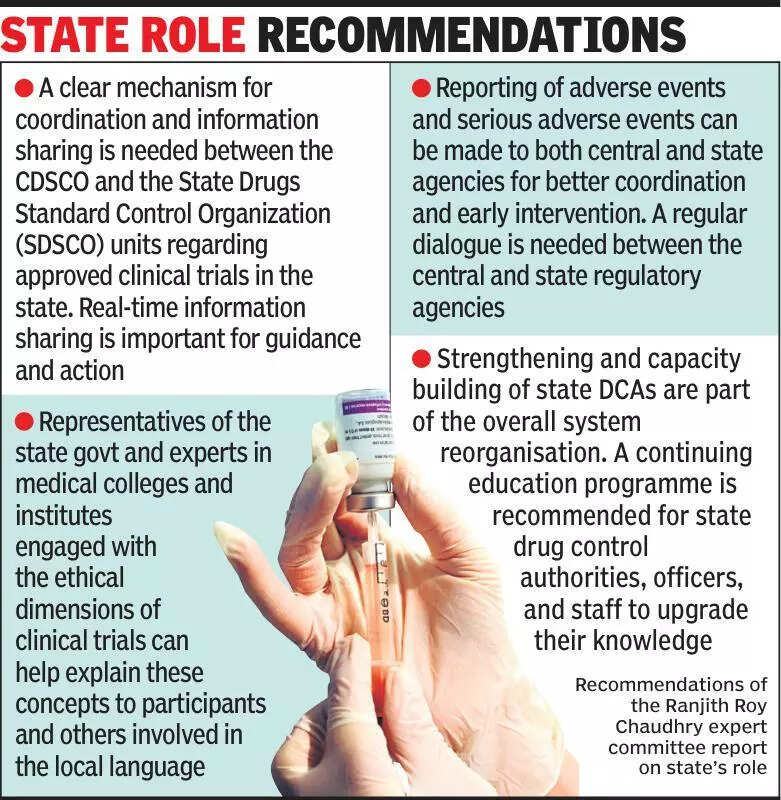
Clinical trials experts have emphasized the importance of keeping states informed about ongoing clinical trials to ensure transparency and adherence to regulations. In a recent discussion, experts highlighted the need for effective communication between central regulatory bodies and state governments to streamline the process of conducting clinical trials in India. The experts pointed out that involving state governments in the decision-making process can help in addressing any concerns or challenges faced during the trials. It was also noted that states play a crucial role in ensuring the safety and well-being of participants in clinical trials. By keeping states informed, regulatory bodies can ensure that trials are conducted ethically and in compliance with all guidelines. Additionally, experts emphasized the need for increased awareness among healthcare professionals and the public about the importance of clinical trials in advancing medical research and improving healthcare outcomes. They stressed the need for collaborative efforts between stakeholders to create a conducive environment for conducting clinical trials in India. Overall, the experts underscored the importance of transparency, communication, and collaboration in the successful conduct of clinical trials in the country.
Posted in
JUST IN
Experts advocate informing states about clinical trials for better oversight and transparency, emphasizing importance for healthcare.
In Trend

Kaleshwaram Project Ex-ENC Arrested for Disproportionate Assets in Hyderabad – Corruption Scandal Unveiled





















