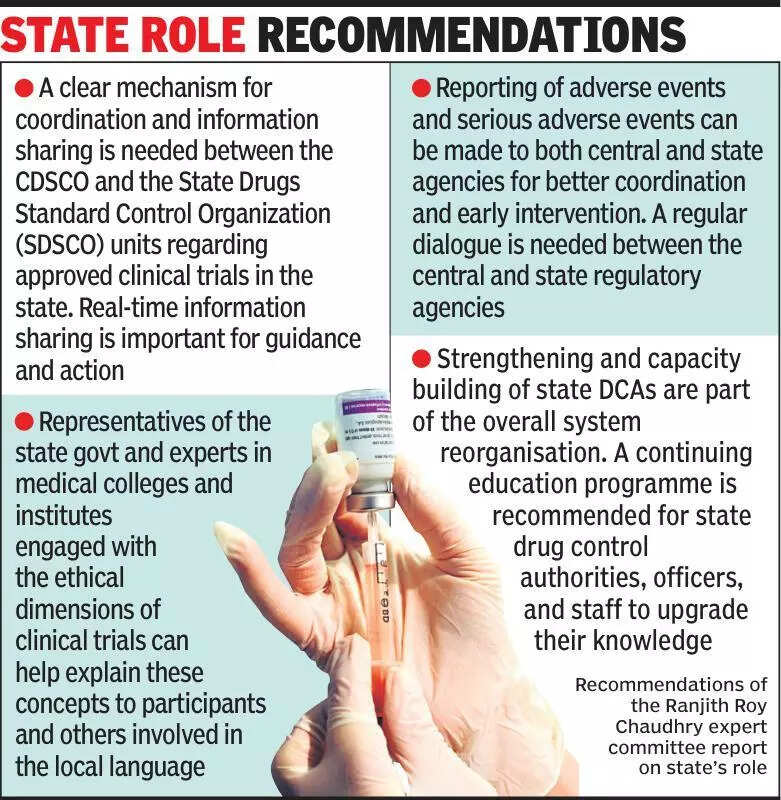
Experts are emphasizing the importance of ensuring that states are informed about clinical trials in India. Clinical trials are essential for the development and approval of new medical treatments and drugs. However, experts believe that states should have more involvement and oversight in the process to ensure the safety and well-being of participants. The lack of transparency and communication between the central government and state governments regarding clinical trials has been a cause for concern. It is crucial for states to be informed about ongoing trials in their respective regions to address any potential issues or concerns that may arise. By keeping states informed, there can be better coordination and monitoring of clinical trials, ultimately leading to improved healthcare outcomes for the public. Experts are calling for greater collaboration between the central government, state governments, and regulatory bodies to establish clear guidelines and protocols for conducting clinical trials in India. This will help ensure that ethical standards are maintained, and participants are protected throughout the trial process. Overall, the involvement of states in clinical trials is seen as a necessary step towards enhancing the regulatory framework and ensuring the safety and efficacy of medical treatments in the country.
Posted in
JUST IN
Experts stress need for states to be informed about clinical trials for better oversight and transparency.
In Trend

“Kaleshwaram Ex-ENC arrested for disproportionate assets in Hyderabad”




















