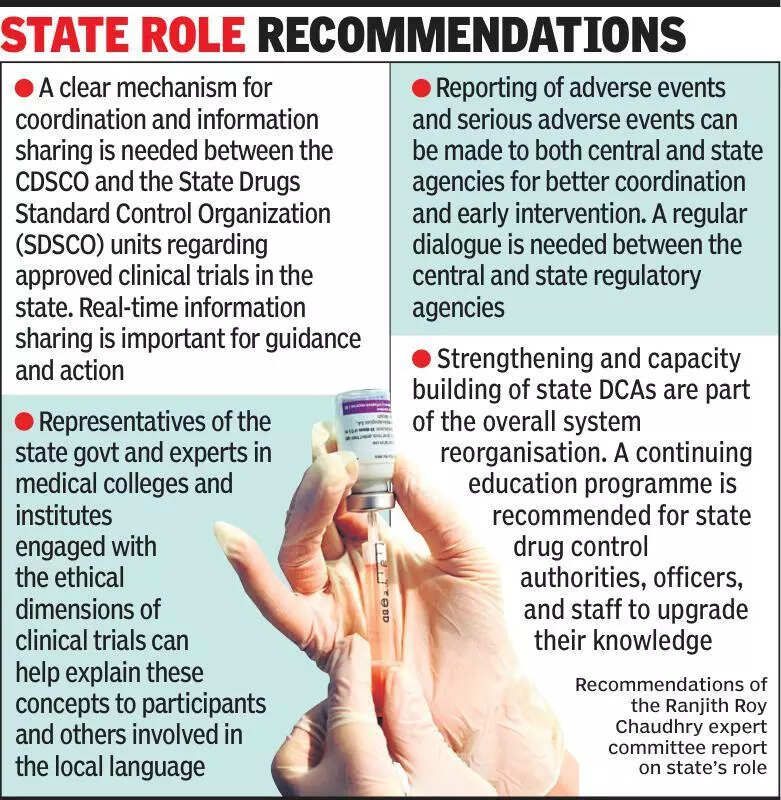
Clinical trials experts are emphasizing the importance of informing states about ongoing clinical trials to ensure transparency and enhance public trust in the process. According to experts, states play a crucial role in overseeing the conduct of clinical trials within their jurisdiction. By keeping states informed about the trials taking place, it not only promotes accountability but also allows for better monitoring of the trials. The lack of communication with states can lead to misunderstandings and distrust among the public, which can ultimately impact the success of the trials. Experts suggest that regular updates and communication with state authorities can help address any concerns or issues that may arise during the course of the trials. This proactive approach can also help in fostering a collaborative environment between researchers, regulatory authorities, and the public. In India, where clinical trials are a vital part of healthcare research, ensuring state involvement and awareness can contribute to a more robust and ethical conduct of trials. By keeping states well-informed about clinical trials, researchers can demonstrate their commitment to upholding the highest standards of research integrity and participant safety. Ultimately, transparent communication with states can lead to more efficient and successful clinical trials, benefiting both the research community and the public.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and accountability in medical research.
In Trend

Gujarat man arrested for threatening Gautam Gambhir: Delhi Police probe ongoing.



















