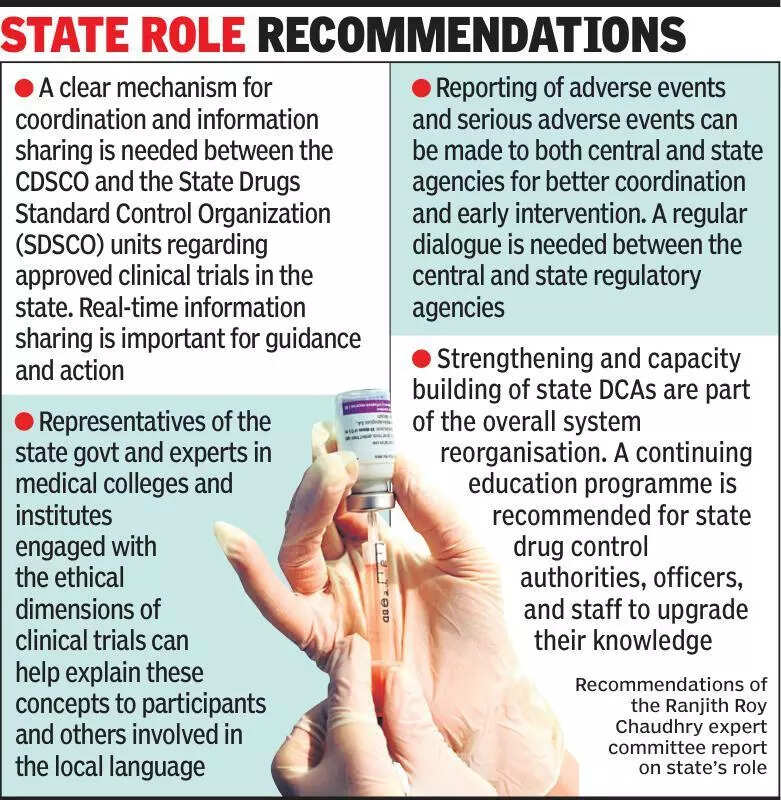
Clinical trials in India are a crucial aspect of the healthcare system, with experts emphasizing the need for states to be more informed about these trials. The process of conducting clinical trials in India has been under scrutiny in recent years, with concerns raised about transparency and ethical practices. Experts believe that involving states in the decision-making process and keeping them informed about ongoing trials is essential to ensure the safety and well-being of participants. By increasing transparency and communication with states, regulatory bodies can better monitor and regulate clinical trials to protect the rights of participants. It is also important for states to be aware of the potential benefits and risks associated with clinical trials, as this knowledge can help them make informed decisions about participating in trials. Overall, experts stress the importance of collaboration between states, regulatory bodies, and healthcare professionals to ensure that clinical trials in India are conducted ethically and transparently. By working together, all stakeholders can help to improve the quality and safety of clinical trials, ultimately benefiting the healthcare system and the Indian population as a whole.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and accountability in healthcare research.
In Trend

Gujarat man arrested for threatening Gautam Gambhir: Delhi Police. Family cites mental health issues. Investigation underway.




















