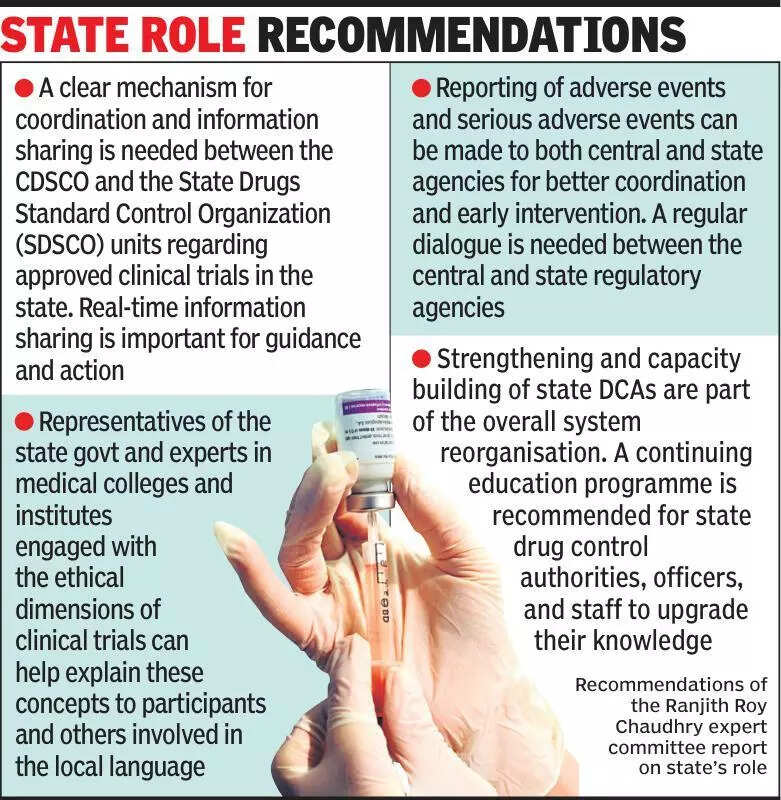
Clinical trials play a crucial role in the development of new medicines and treatments, but experts emphasize the need for transparency and communication with state governments in India. According to experts in the field, it is essential for states to be informed about ongoing clinical trials to ensure accountability and oversight. This communication is vital to address any concerns or issues that may arise during the trial process. Transparency in clinical trials can help build trust with the public and ensure that the trials adhere to ethical standards and regulations. By keeping state governments informed, researchers can also benefit from local expertise and resources that may aid in the successful completion of the trials. It is crucial for all stakeholders involved in clinical trials to work together to ensure the safety and efficacy of new treatments. Additionally, clear communication with state governments can help streamline the regulatory process and facilitate the timely approval of new medications. Overall, experts stress the importance of collaboration and transparency in clinical trials to advance medical research and improve healthcare outcomes in India.
Posted in
JUST IN
Experts urge states to be kept informed about clinical trials for improved transparency and oversight.
In Trend

Gujarat man arrested for threatening Gautam Gambhir; family cites mental health concerns. Delhi Police investigate.