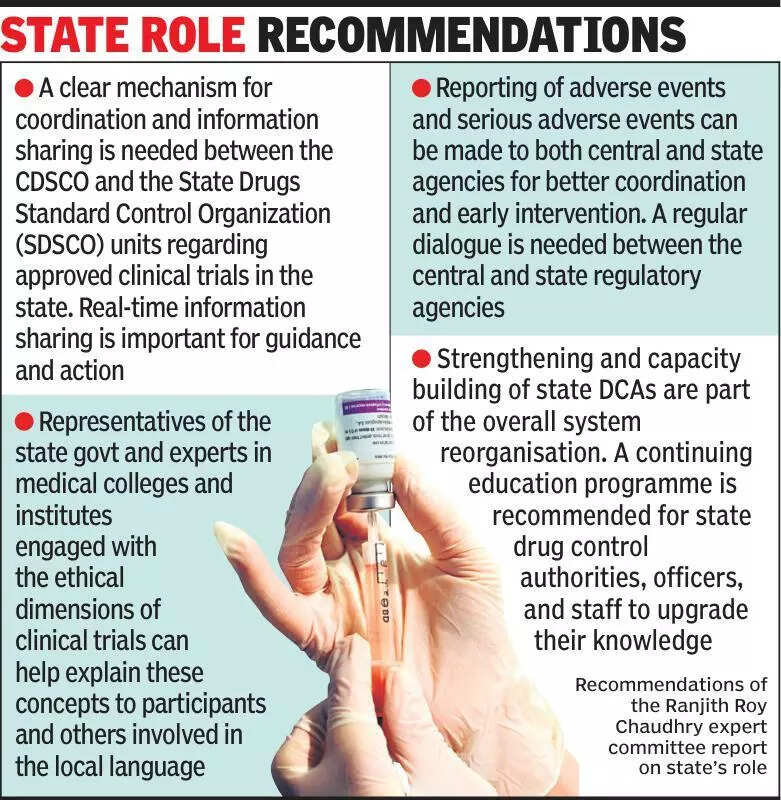
Health experts are emphasizing the importance of informing states about clinical trials to ensure transparency and accountability in the process. Clinical trials play a crucial role in the development of new drugs and treatments, making it essential for states to be kept in the loop. By involving states in the process, it can help in monitoring the trials closely and ensuring the safety and well-being of participants. Experts believe that this collaboration can also help in addressing any ethical concerns that may arise during the trials. The involvement of states can also help in creating a more robust regulatory framework for clinical trials in India. With the increasing number of clinical trials being conducted in the country, it is crucial to have a strong regulatory mechanism in place to protect the interests of participants and ensure the credibility of the trials. By keeping states informed about clinical trials, it can lead to better coordination between all stakeholders involved in the process. This can ultimately result in more effective and efficient clinical trials that adhere to the highest ethical standards. It is essential for all stakeholders, including pharmaceutical companies, research institutions, and regulatory bodies, to work together to ensure that clinical trials are conducted ethically and transparently. This collaborative approach can help in advancing medical research and improving healthcare outcomes for the people of India.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and accountability in healthcare research.
In Trend

Gujarat man arrested for threatening emails to Gambhir: Delhi Police. Mental health issues claimed. Investigation ongoing.





















