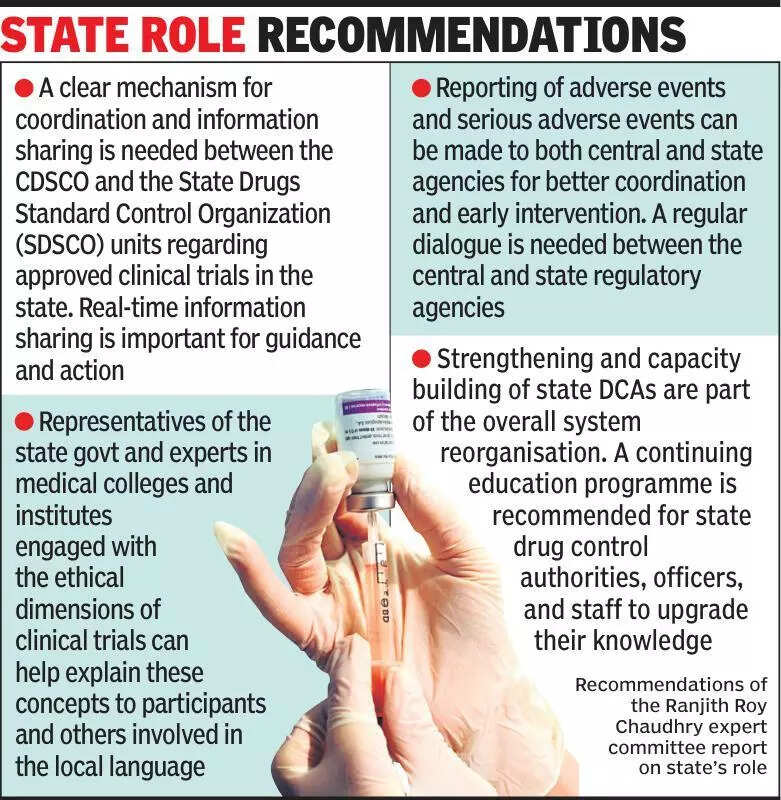
Experts in the field of clinical trials have emphasized the importance of keeping states informed about ongoing research to ensure transparency and adherence to regulations. In India, where clinical trials play a crucial role in the pharmaceutical industry, experts assert that state governments should be actively involved in overseeing these trials to safeguard the interests of participants. The involvement of state authorities can help in monitoring the ethical conduct of trials, ensuring the safety of participants, and maintaining compliance with regulatory guidelines. By keeping states informed about clinical trials, researchers can also benefit from valuable input and feedback, which can enhance the quality and credibility of the research. It is essential for all stakeholders involved in clinical trials to collaborate effectively to uphold the highest standards of ethics and safety. The active participation of state governments can contribute to building trust and confidence in the clinical trial process among the public. Additionally, involving states in the oversight of clinical trials can help in addressing any concerns or issues that may arise during the research process. Overall, experts believe that transparency and collaboration between researchers and state authorities are essential for the successful conduct of clinical trials in India. This approach can not only benefit the participants and researchers but also contribute to the advancement of medical science and healthcare in the country.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and accountability in healthcare system.
In Trend

Gujarat man arrested for threatening Gambhir: Delhi Police. Family cites mental health issues. Ongoing investigation.