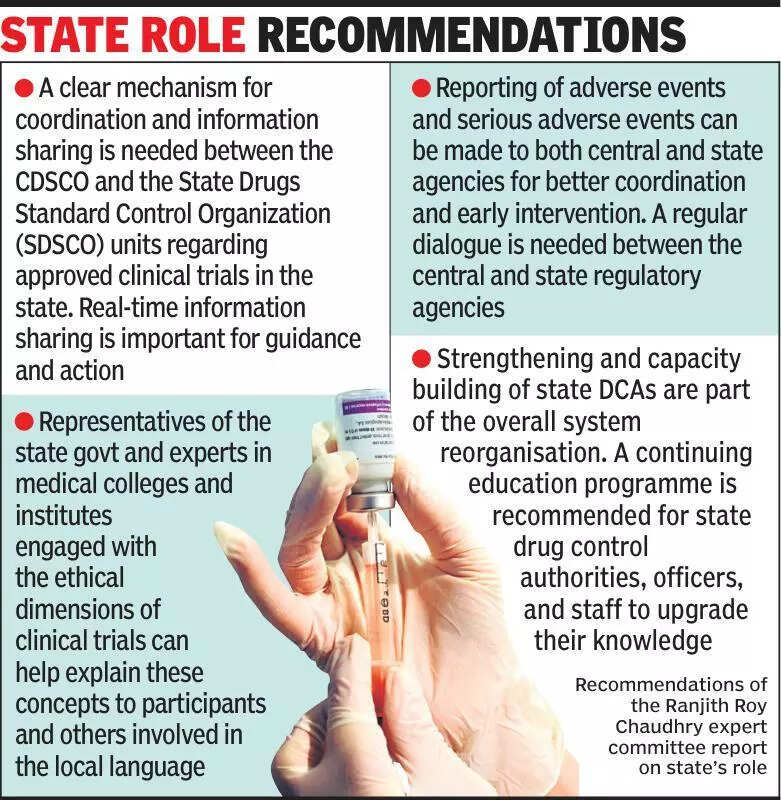
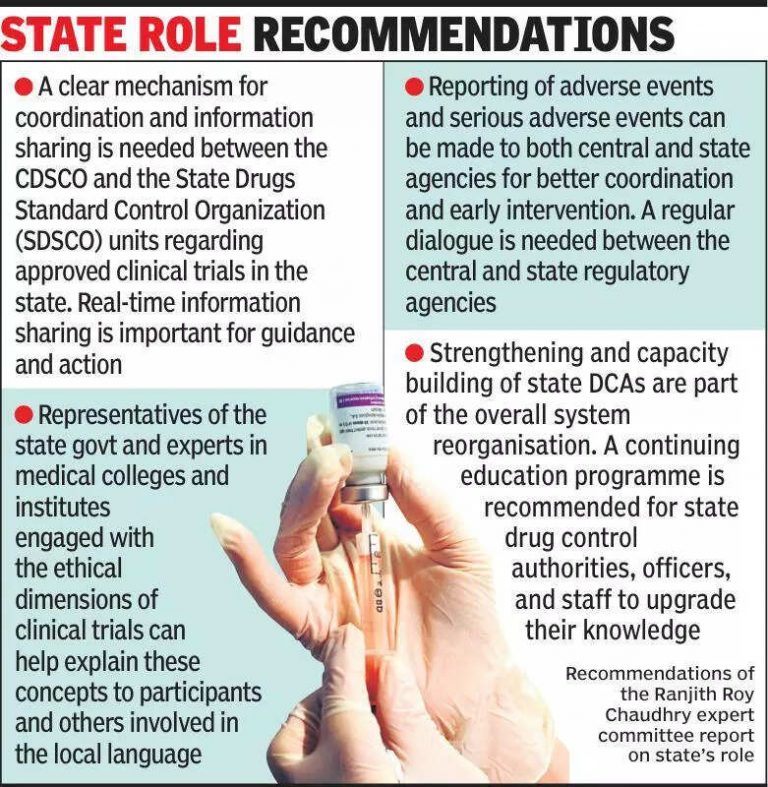
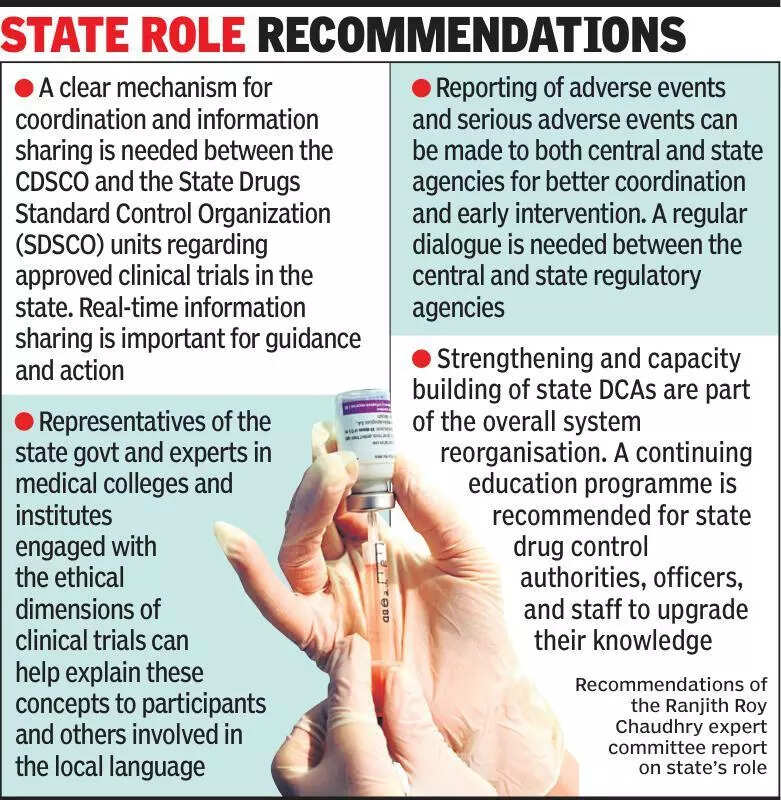
Experts are emphasizing the importance of informing states about clinical trials to ensure transparency and adherence to regulations. Clinical trials play a crucial role in the development of new medical treatments and drugs. However, experts are concerned about the lack of communication between the central government and state authorities regarding these trials. By keeping states informed, experts believe that the entire process can be more efficient and effective. It is essential for states to be aware of the clinical trials taking place within their jurisdiction to monitor any potential risks and ensure that ethical standards are being followed. This collaboration between the central government and states can lead to better oversight and regulation of clinical trials. With India being a hub for clinical research, it is imperative to establish clear communication channels between all stakeholders involved. By keeping states informed about clinical trials, the country can continue to contribute to medical advancements while upholding the highest standards of patient safety and ethical practices. Stay updated on the latest developments in clinical trials and medical research by following reputable sources and staying informed about the regulatory landscape in India.
Posted in
JUST IN
“Experts Call for State Involvement in Clinical Trials for Better Transparency and Regulation”
In Trend

Telangana High Court orders analogous hearing in Ghatkesar land dispute, ensuring fair legal proceedings.