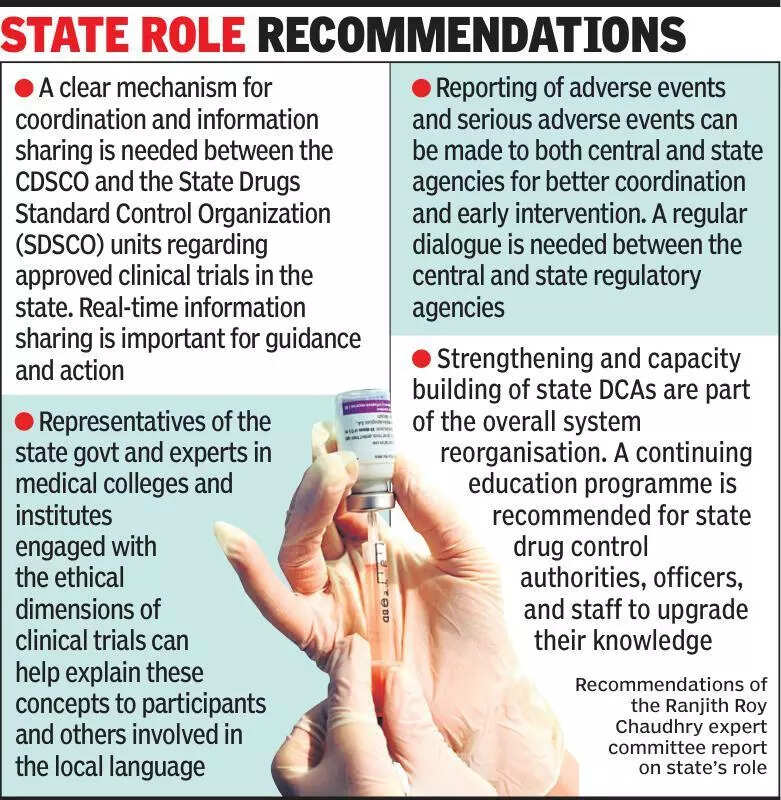
Clinical trials in India have been a topic of discussion among experts, who believe that states should be more informed about these trials. The importance of transparency and communication between the central government and state authorities regarding clinical trials has been emphasized by these experts. This discussion comes in light of the recent controversy surrounding the approval and conduct of clinical trials in the country. It is crucial for all stakeholders involved in clinical trials to maintain open channels of communication and ensure that proper protocols are followed to protect the safety and rights of participants. The need for increased awareness and education about clinical trials among the general public has also been highlighted. Experts suggest that more efforts should be made to inform people about the purpose, process, and potential risks and benefits of participating in clinical trials. By increasing awareness and transparency, the overall quality of clinical trials in India can be improved, leading to better healthcare outcomes for the population. It is essential for regulatory bodies, researchers, and healthcare providers to work together to ensure that clinical trials are conducted ethically and in compliance with all regulations. Overall, the call for better communication and transparency in clinical trials is aimed at promoting trust and confidence in the healthcare system in India.
Posted in
JUST IN
Experts emphasize informing states about clinical trials for transparency and accountability in medical research.
In Trend

Gujarat man arrested for threatening Gautam Gambhir: Delhi Police. Family cites mental health concerns. Ongoing investigation.

















