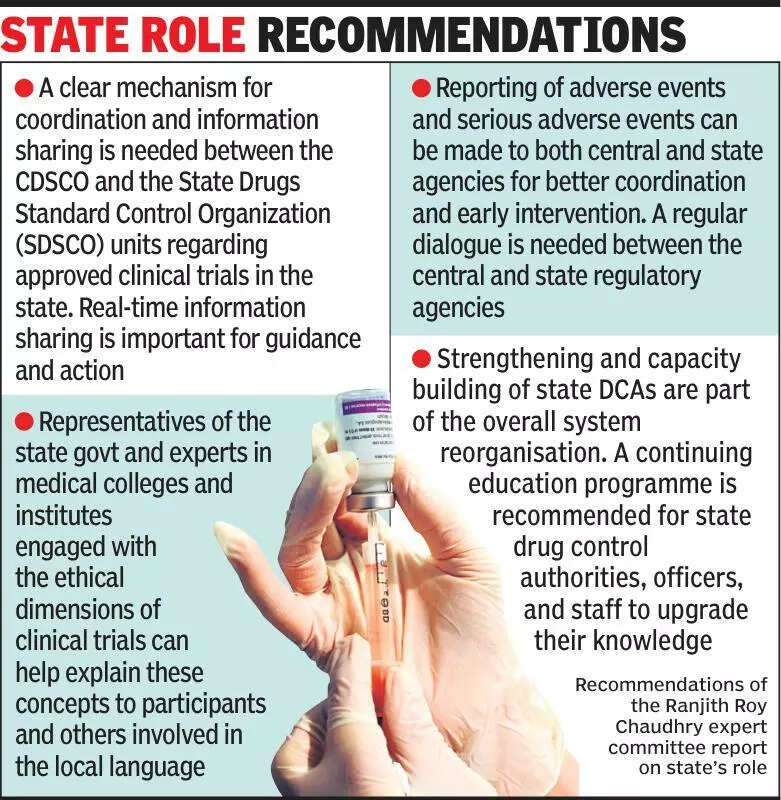
Experts in the medical field have emphasized the importance of informing states about clinical trials to ensure transparency and accountability in the process. Clinical trials are crucial for testing the safety and efficacy of new drugs and treatments before they are made available to the public. However, there have been concerns about the lack of communication between the central government and state governments regarding these trials. Experts have pointed out that states play a significant role in facilitating and monitoring clinical trials within their jurisdictions. By keeping states informed about the trials taking place in their states, the government can ensure better oversight and regulation of the process. This will also help in addressing any issues or concerns that may arise during the trials. It is essential for all stakeholders to work together to streamline the process and ensure that the highest standards of ethics and safety are maintained. By improving communication and collaboration between the central government and states, India can strengthen its position as a leading hub for clinical research. This will not only benefit the healthcare industry but also pave the way for the development of innovative treatments and therapies. In conclusion, experts stress the need for increased transparency and cooperation to enhance the effectiveness of clinical trials in India.
Posted in
JUST IN
Experts emphasize notifying states about clinical trials for transparency and oversight in research processes.
In Trend

Kaleshwaram project ex-ENC arrested for possessing disproportionate assets, Hyderabad.