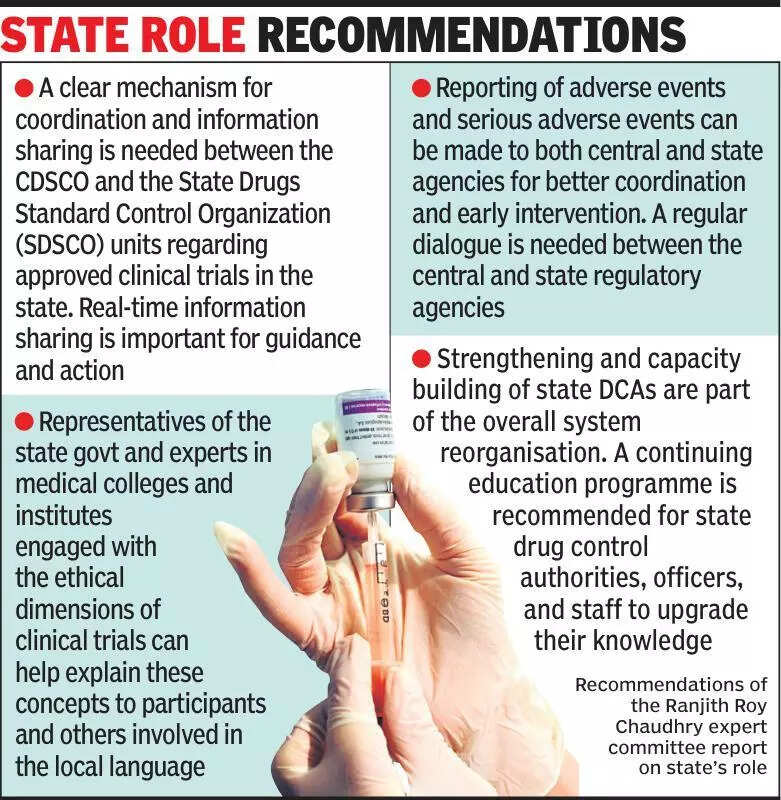
Experts are emphasizing the importance of keeping states informed about clinical trials in India. Clinical trials play a crucial role in the development of new drugs and treatments, and it is essential for states to be aware of these trials to ensure proper monitoring and regulation. This helps in maintaining the safety and effectiveness of the trials. The experts have pointed out that states have an important role to play in overseeing clinical trials conducted within their jurisdictions. By staying informed about these trials, states can ensure that ethical guidelines are followed, and the rights of participants are protected. Additionally, being informed about clinical trials allows states to monitor the progress of research and its impact on public health. Experts have also highlighted the need for greater collaboration between states and the central government to streamline the process of approving and monitoring clinical trials. This collaboration can help in ensuring that trials are conducted efficiently and transparently. Overall, keeping states informed about clinical trials is essential for promoting ethical research practices and safeguarding the well-being of participants.
Posted in
JUST IN
Experts stress informing states about clinical trials for better oversight and regulation, highlighting importance for transparency.
In Trend

Gujarat man arrested for threatening Gautam Gambhir; Delhi Police investigates mental health claims.




















