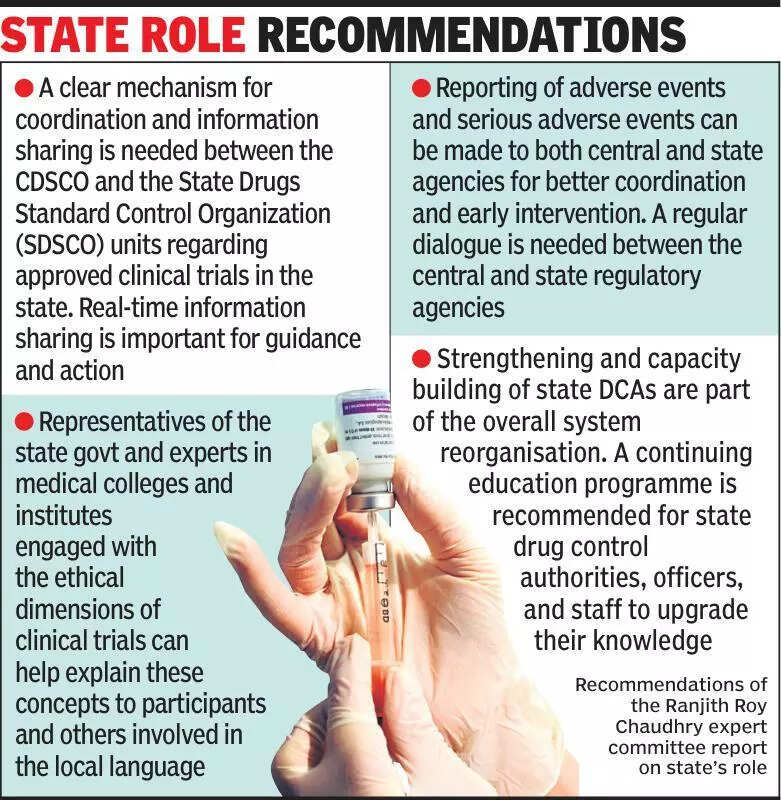
Clinical trials are a crucial aspect of the healthcare industry in India, and experts emphasize the importance of keeping states informed about these trials. According to experts, transparency and communication with state governments are essential to ensure the smooth conduct of clinical trials. The lack of information sharing can lead to misunderstandings and delays in the approval process. It is vital for pharmaceutical companies and research organizations to engage with state authorities to provide updates on the progress of clinical trials and address any concerns they may have. By establishing clear channels of communication, stakeholders can work together effectively to advance medical research while adhering to regulatory requirements. Additionally, involving states in the decision-making process can help build trust and foster collaboration in the healthcare sector. Overall, experts stress the need for greater transparency and collaboration between all parties involved in clinical trials to promote patient safety and ensure the success of medical research in India.
Posted in
JUST IN
Experts stress informing states about clinical trials for better oversight and transparency, enhancing healthcare quality.
In Trend

Kaleshwaram project ex-ENC arrested for disproportionate assets in Hyderabad.





















