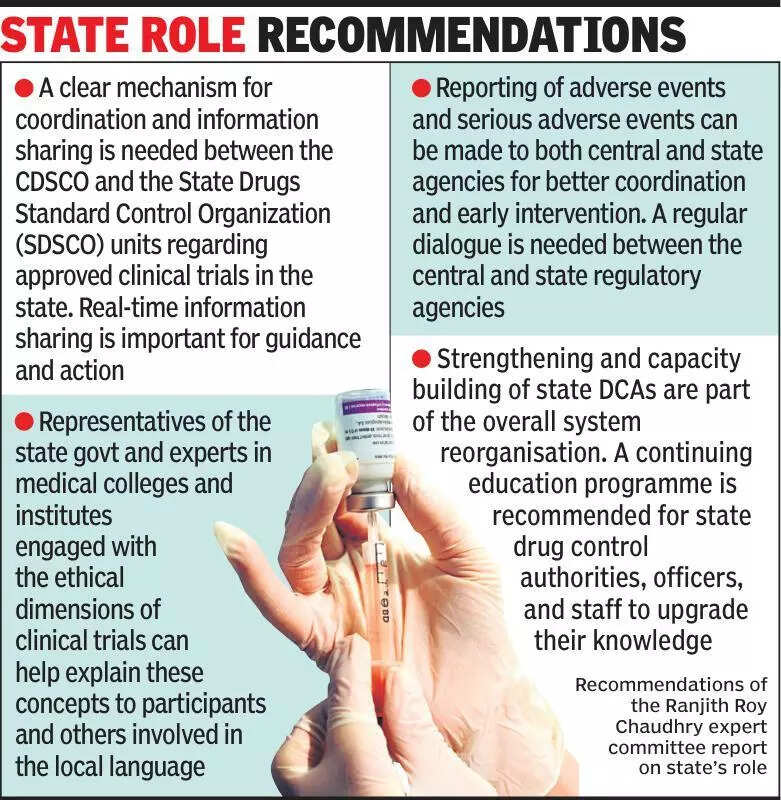
Clinical trials experts emphasize the importance of informing states about ongoing clinical trials to ensure transparency and accountability in the process. In a recent discussion, experts highlighted the need for better communication between the central government and state authorities regarding clinical trials being conducted in India. The lack of information flow can lead to misunderstandings and delays in the approval process, ultimately affecting the timely completion of trials. It is crucial for states to be aware of all trials taking place within their jurisdiction to address any concerns related to safety, ethics, or compliance with regulations. By keeping states informed, the regulatory bodies can work together more efficiently to oversee and monitor clinical trials, safeguarding the interests of participants and upholding the integrity of the research. Additionally, increased transparency can help build trust among the public and encourage more people to participate in clinical trials, ultimately advancing medical research in the country. Experts suggest establishing clear communication channels and protocols for sharing information between the central and state governments to streamline the regulatory process. Overall, a collaborative approach between all stakeholders is essential to ensure the success and credibility of clinical trials in India.
Posted in
JUST IN
Experts stress informing states about clinical trials for better transparency and oversight in medical research.
In Trend

Gujarat man arrested for threatening Gambhir: Delhi Police. Mental health concerns raised. Investigation underway.


















