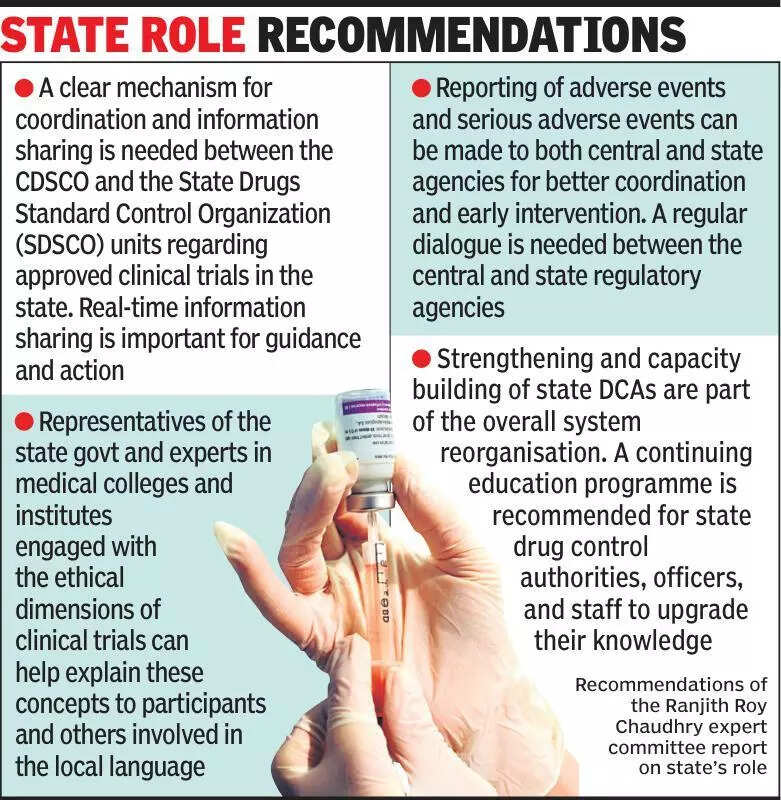
Experts are emphasizing the importance of informing states about clinical trials to ensure transparency and compliance with regulations in India. Clinical trials play a crucial role in the development of new drugs and treatments, but there have been concerns about ethical practices and patient safety. By keeping states informed about ongoing trials, authorities can monitor the process closely and ensure that all necessary protocols are being followed. This will help in maintaining the integrity of the trials and protecting the rights of participants. Additionally, sharing information with states can enhance collaboration between different stakeholders and improve the overall quality of research. It is essential for pharmaceutical companies and research institutions to work closely with state governments to create a robust regulatory framework for clinical trials. This will not only benefit the healthcare system but also contribute to the advancement of medical science in the country. By prioritizing transparency and communication, India can establish itself as a leader in ethical and high-quality clinical research.
Posted in
JUST IN
Experts stress informing states about clinical trials for improved transparency and oversight in healthcare research.
In Trend

Gujarat man arrested for sending threatening emails to Gautam Gambhir; Delhi Police investigates mental health claims.


















