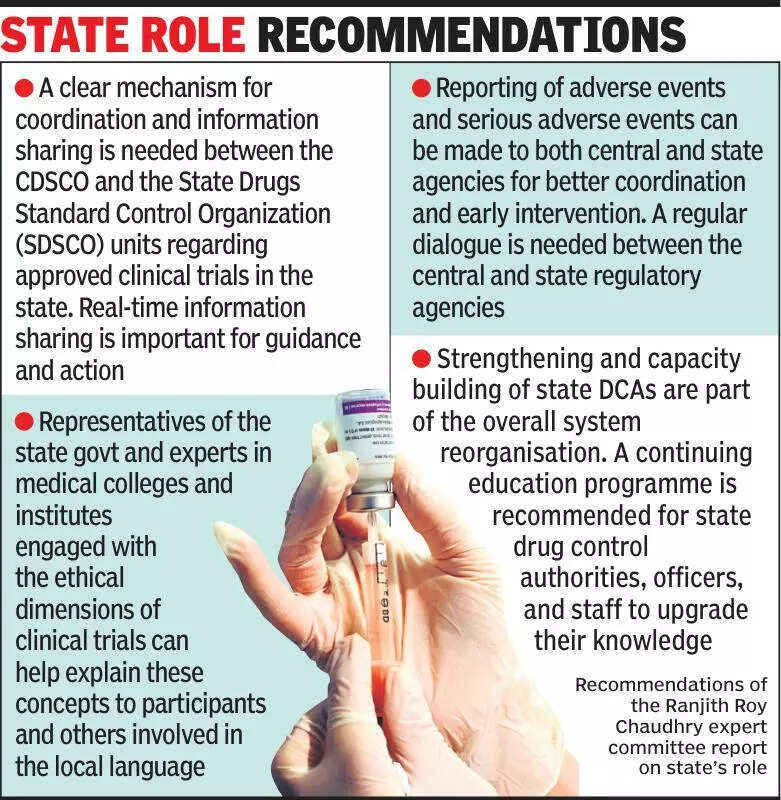
Clinical trials experts emphasize the importance of informing states about ongoing clinical trials to ensure better transparency and monitoring. In a recent discussion, experts highlighted the need for states to be actively involved in the process to uphold ethical standards and safeguard participants’ rights. The call for increased communication comes in light of concerns raised about the lack of information sharing between trial sponsors and state governments. By involving states in the loop, experts believe that there will be enhanced oversight and accountability throughout the trial process. This move is crucial in maintaining public trust and confidence in the clinical trial system. Additionally, experts stress the significance of robust regulatory mechanisms to oversee and regulate clinical trials effectively. They suggest that a collaborative approach between all stakeholders, including states, sponsors, and regulatory bodies, is essential to ensure that trials are conducted ethically and adhere to all guidelines and protocols. Increased transparency and communication are key factors in addressing any potential issues and ensuring that trials are conducted in a fair and ethical manner. As India continues to emerge as a hub for clinical research, it is imperative to strengthen regulatory frameworks and foster cooperation between all parties involved. By fostering a culture of openness and collaboration, the clinical trial ecosystem in India can thrive, benefiting both researchers and participants alike.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and collaboration in healthcare advancements.
In Trend

Kaleshwaram project ex-ENC arrested for possessing disproportionate assets, faces corruption charges in Hyderabad.





















