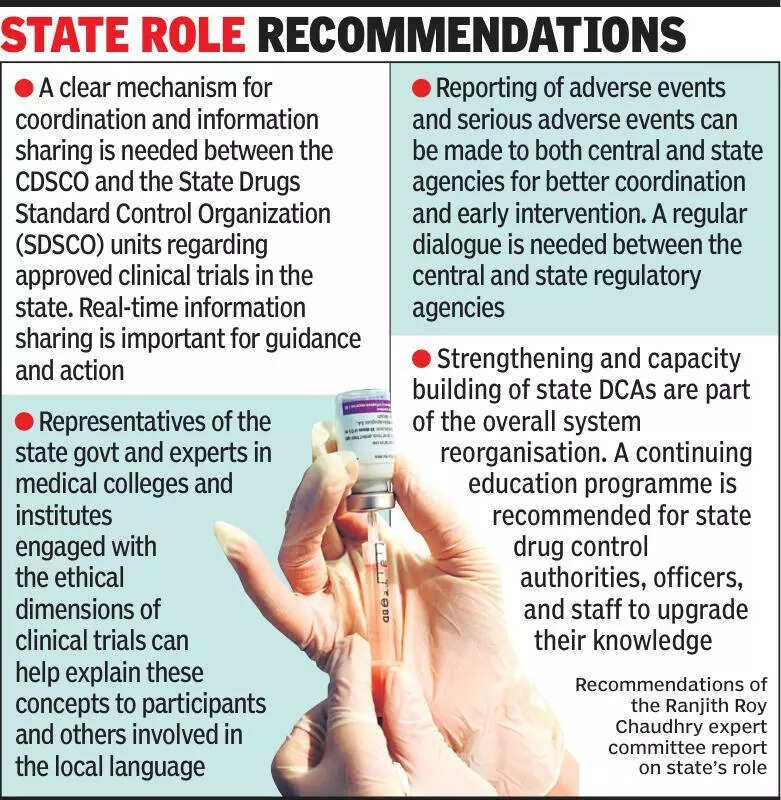
Experts are emphasizing the importance of informing states about clinical trials in India. Clinical trials are essential for testing the safety and efficacy of new drugs and treatments. However, experts believe that states should be kept in the loop to ensure transparency and accountability. The lack of communication with states can lead to misunderstandings and confusion about the purpose and outcomes of clinical trials. It is crucial for all stakeholders, including pharmaceutical companies, research institutions, and regulatory bodies, to involve states in the decision-making process regarding clinical trials. By keeping states informed, the overall process can be more streamlined and efficient. This approach can also help build trust and collaboration between different parties involved in clinical trials. Ultimately, the goal is to ensure that clinical trials are conducted ethically and with the best interests of patients in mind. Experts are urging for better communication and coordination between all stakeholders to improve the clinical trial landscape in India. Stay updated with the latest news and developments in clinical trials by following reputable sources like Times of India.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and collaboration, promoting better healthcare outcomes.
In Trend

Gujarat man arrested for threatening Gautam Gambhir; Delhi Police investigating mental health angle.




















