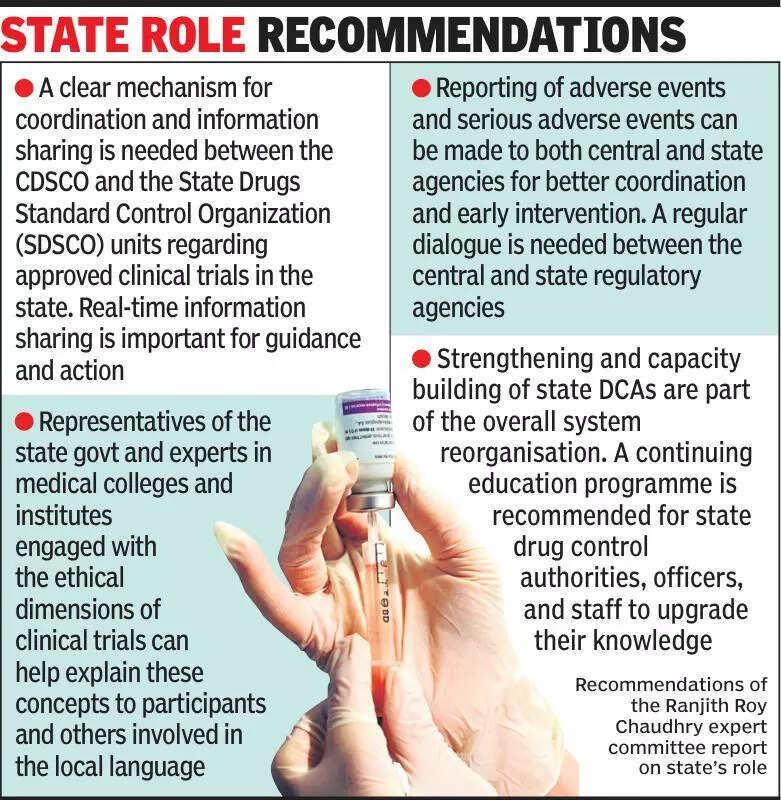
Experts are emphasizing the importance of keeping states informed about clinical trials in India. Clinical trials are crucial for testing the safety and efficacy of new drugs and treatments. However, experts believe that states should be more involved in the process to ensure transparency and accountability. The lack of communication between the central government and states can lead to misunderstandings and delays in the approval process. By keeping states informed about clinical trials, it can help in better coordination and implementation of regulations. This will ultimately benefit patients by ensuring that they have access to safe and effective treatments. Experts also suggest that states should have a more active role in monitoring and overseeing clinical trials to protect the interests of participants. In conclusion, better communication and collaboration between the central government and states are essential for the successful conduct of clinical trials in India.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and oversight, crucial for healthcare advancements.
In Trend

Former ENC of Kaleshwaram project arrested for owning disproportionate assets, facing corruption charges in Hyderabad.






