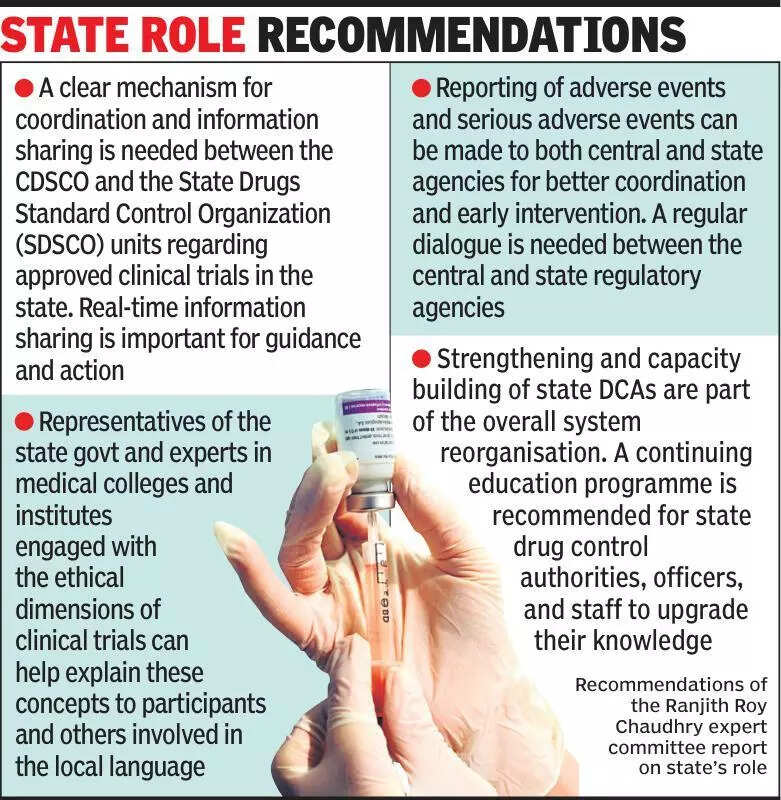
Experts in the field of healthcare are emphasizing the importance of informing states about clinical trials to ensure transparency and accountability in the process. Clinical trials play a crucial role in the development of new medical treatments and medications, making it essential for states to be kept informed. By involving states in the process, there is better oversight and regulation, ultimately leading to improved healthcare outcomes for the population. The need for transparency in clinical trials is particularly important in a country like India, where the pharmaceutical industry is rapidly growing. Involving states in the decision-making process can help ensure that the interests of the population are protected and that ethical standards are maintained. It also allows for better monitoring of any adverse effects that may arise during the trials. Experts believe that by keeping states informed about clinical trials, the healthcare system as a whole can benefit from the knowledge and insights gained. This collaboration between the pharmaceutical industry, healthcare providers, and state governments can lead to more effective and safer medical treatments being developed. It is crucial for all stakeholders to work together to ensure that clinical trials are conducted ethically and with the best interests of the population in mind. By prioritizing transparency and communication, the healthcare industry in India can continue to grow and innovate while also safeguarding the well-being of its citizens.
Posted in
JUST IN
Experts stress informing states about clinical trials for transparency and oversight in medical research.
In Trend

Kaleshwaram project ex-ENC arrested for owning assets disproportionate to income in Hyderabad.




















