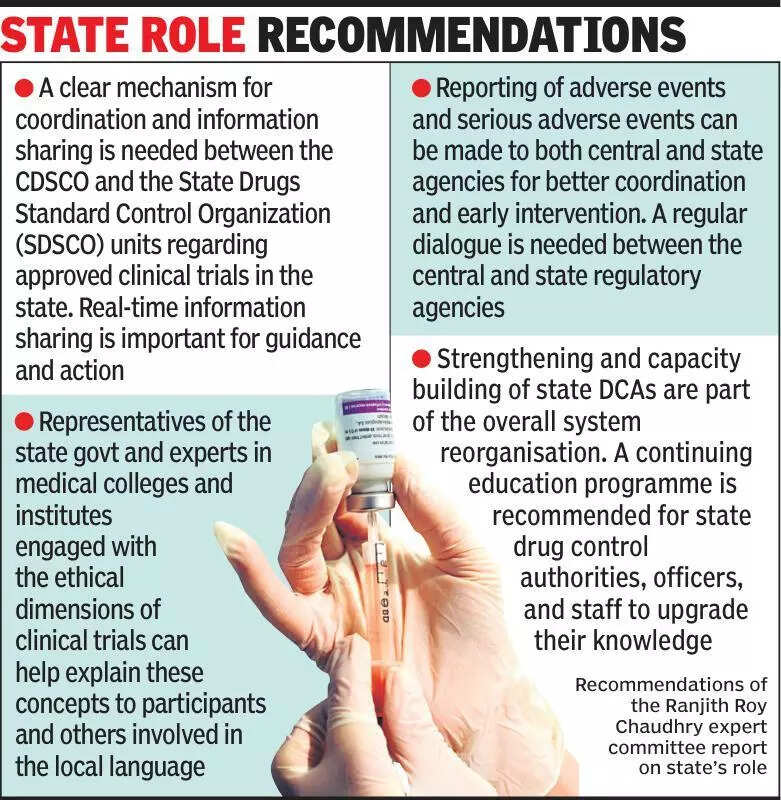
Experts in the medical field are emphasizing the importance of keeping states informed about clinical trials in India. Clinical trials play a crucial role in testing the effectiveness and safety of new medications and treatments. However, experts are highlighting the need for better communication and transparency between researchers conducting clinical trials and state governments. By keeping states informed about the ongoing trials, they can ensure that the necessary regulatory and ethical guidelines are being followed. This will help in maintaining the integrity of the trials and ultimately lead to better healthcare outcomes for the Indian population. It is essential for researchers to collaborate with state authorities to ensure that the trials are conducted in a responsible and ethical manner. By fostering this collaboration, India can continue to be a key player in the global pharmaceutical industry. In recent years, India has emerged as a hub for clinical trials, attracting researchers and pharmaceutical companies from around the world. However, to sustain this growth and reputation, it is crucial to prioritize transparency and communication with state governments. This will not only benefit the healthcare sector but also contribute to India’s overall development as a leader in medical research and innovation. Moving forward, experts are advocating for increased dialogue and collaboration between researchers, pharmaceutical companies, and state authorities to ensure that clinical trials in India are conducted with the highest standards of ethics and safety. By working together, stakeholders can pave the way for groundbreaking discoveries and advancements in healthcare that will benefit the entire nation.
Posted in
JUST IN
Experts stress informing states about clinical trials to improve transparency and oversight in healthcare research.
In Trend

Former ENC of Kaleshwaram project arrested for possessing disproportionate assets, faces legal action in Hyderabad.