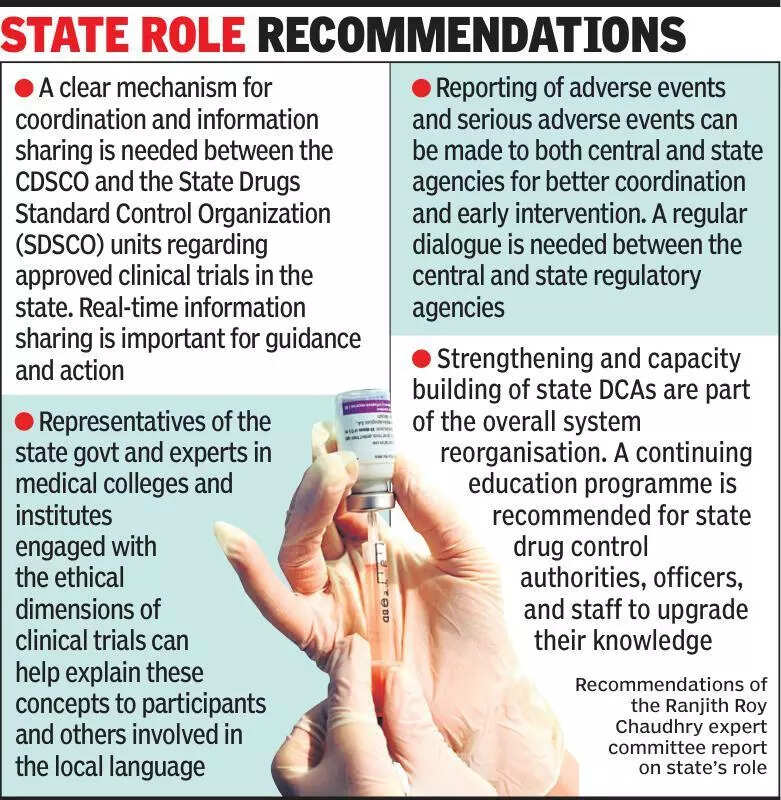
Clinical trials experts emphasize the importance of keeping states informed about ongoing clinical trials to ensure transparency and accountability in the process. In a recent discussion, experts highlighted the need for better communication between the central government, states, and various stakeholders involved in clinical trials. This move aims to address concerns related to the lack of awareness and information about clinical trials, which can lead to misunderstandings and misinformation. By keeping states informed about the progress and outcomes of clinical trials, experts believe that transparency and trust can be maintained, ultimately benefiting the healthcare system as a whole. Additionally, experts stressed the importance of adhering to ethical guidelines and regulations set by regulatory bodies to safeguard the rights and well-being of trial participants. This discussion comes in the wake of increasing interest and participation in clinical trials in India, highlighting the need for a more structured and transparent approach to ensure the safety and efficacy of new treatments. As India emerges as a hub for clinical research, experts urge the government and stakeholders to prioritize communication and information sharing to build a strong foundation for conducting ethical and successful clinical trials in the country. Stay updated on the latest developments and insights in the field of clinical trials by following reputable sources and staying informed about ongoing research initiatives in India.
Posted in
JUST IN
Experts stress informing states on clinical trials for transparency and safety, urging collaboration for ethical research.
In Trend

Gujarat man arrested for threatening Gautam Gambhir; family cites mental health issues in Delhi Police investigation.
















