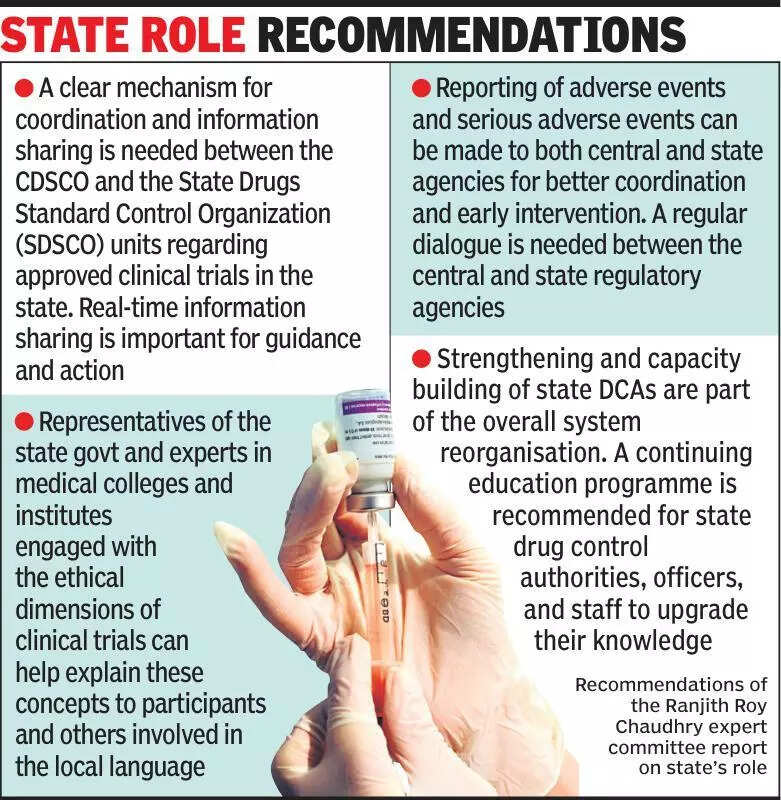
Experts have emphasized the importance of keeping states informed about clinical trials to ensure transparency and better monitoring of the process. Clinical trials play a crucial role in the development of new medical treatments and drugs. However, there have been instances where lack of communication and coordination between states and central government has led to challenges in monitoring these trials effectively. By keeping states informed about the trials taking place within their jurisdiction, it can help in ensuring compliance with regulations and ethical standards. This will also enable the states to provide necessary support and oversight to the trials. Experts have also highlighted the need for regular communication and sharing of information between the parties involved in clinical trials to avoid any discrepancies or delays. Transparency and collaboration are key in ensuring the success and safety of clinical trials. With the right mechanisms in place, states can play a more active role in monitoring and regulating clinical trials to protect the interests of participants and uphold ethical standards. It is essential for all stakeholders to work together towards a common goal of advancing medical research while maintaining high standards of ethics and patient safety.
Posted in
JUST IN
Experts stress the need for states to be informed about clinical trials for better regulation and oversight.
In Trend

Gujarat man arrested for threatening Gautam Gambhir; mental health issues raised. Delhi Police investigating.




















